Introduction
The vascular disrupting agent OXi4503 induces apoptosis in malignant myeloblasts by removing endothelial cell protection and sensitizing blasts to the cytotoxic effects of cytarabine by inducing them into the cell cycle (PMID 26898708). Cogle et al confirmed the safety and feasibility of single agent intravenous (IV) OXi4503 up to doses of 7.81 mg/m2 (maximum tolerated dose [MTD] not reached) in patients with relapsed/refractory acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Based on these data and pre-clinical evidence of synergy in vitro, a Phase 1b dose-finding study was initiated to evaluate the safety and tolerability of combined therapy with OXi4503 and cytarabine for a similar patient population.
Methods
The primary objective of this study (NCT02576301) was to determine the MTD of OXi4503 in combination with cytarabine in patients with relapsed/refractory AML or MDS. Pharmacokinetic (PK)/pharmacodynamic (PD) studies and a preliminary assessment of efficacy were secondary endpoints. Escalating doses of OXi4503 (in 5 cohorts starting from 3.75 mg/m2 and ending at 9.76 mg/m2) were administered IV over 10 minutes on Days 1 and 4. Fixed dose cytarabine (1 g/m2/day) was administered IV over 2 hours on Days 1-5 and on Days 1 and 4 cytarabine was administered 4 hours after the end of OXi4503 infusion. Cycles were repeated every 28 days for up to 4 cycles.
Results
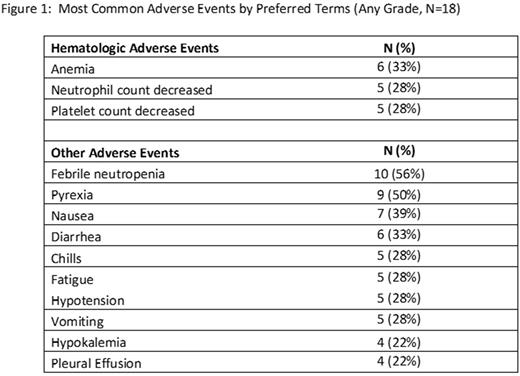
A total of 22 patients (18 currently evaluable) with relapsed/refractory AML were enrolled, with Cohort 5 (9.76 mg/m2) in active treatment at time of writing. Median age was 61 years (range 26 - 77) and 67% were male; 83% of subjects had ECOG status 1 and 17% had ECOG status 2. Subjects had failed a median of 3 (range: 1-8) prior therapies and all had intermediate or poor-risk cytogenetics. Most patients (61%) received 1 cycle of investigational therapy while 39% of patients received 2 or more cycles. 11 of 18 patients (61%) withdrew from the study for progressive disease (PD), 4 of 18 patients (22%) withdrew with persistent AML, and 3 of 18 evaluable patients (17%) achieved a complete remission (CR). An additional 3 patients experienced reduction in bone marrow blasts but did not achieve a CR. CRs were durable for 12 months, 5 months, and one patient remains in ongoing remission for 5 months as of July 2017. One patient in the 3.75 mg/m2 cohort experienced a dose-limiting toxicity of hypofibrinogenemia, but with no clinical evidence of bleeding. Following correction with cryoprecipitate, fibrinogen returned to normal and remained normal unsupported. The most common Grade 3/4 treatment-related adverse events included neutrophil count decreased (28%), platelet count decreased (28%), febrile neutropenia (22%), anemia (17%), and WBC count decreased (11%). Adverse Events of all Grades are presented in Figure 1. Changes in coagulation parameters were common, with 14 of 18 (78%) patients experiencing one or more of: increase in D-Dimer, decrease in fibrinogen, INR or PTT increased. Clinically significant bleeding was not observed. The MTD has not yet been reached on this study.
Conclusions
The vascular disrupting agent OXi4503 in combination with cytarabine (1 g/m2/day) is well tolerated with preliminary evidence of activity in heavily pretreated, relapsed and refractory AML patients, including those with poor risk cytogenetic profiles. This Phase 1b study at time of writing showed evidence of activity at doses from 3.75 to 9.76 mg/m2 with 17% of patients achieving CR, suggesting a re-sensitizing effect to cytarabine. Additional studies of Oxi4503 in well-controlled clinical trials to further characterize the initial safety and efficacy of this novel agent are warranted.
Watts: Jazz Pharmaceuticals: Consultancy, Speakers Bureau. Cogle: Celgene: Other: Membership on Steering Committee for Connect MDS/AML Registry. Schiller: bluebird bio: Research Funding; mateon therapeutics: Research Funding. Lin: Jazz Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal